what happens after damage to the suprachiasmatic nucleus itself?​
| Suprachiasmatic nucleus | |
|---|---|
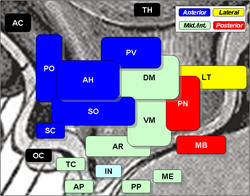 Suprachiasmatic nucleus is SC, at middle left, labelled in bluish. | |
 Suprachiasmatic nucleus is labelled and shown in green. | |
| Details | |
| Identifiers | |
| Latin | nucleus suprachiasmaticus |
| MeSH | D013493 |
| NeuroNames | 384 |
| NeuroLex ID | birnlex_1325 |
| TA98 | A14.i.08.911 |
| TA2 | 5720 |
| FMA | 67883 |
| Anatomical terms of neuroanatomy [edit on Wikidata] | |
The suprachiasmatic nucleus or nuclei (SCN) is a tiny region of the brain in the hypothalamus, situated directly higher up the optic chiasm. It is responsible for controlling circadian rhythms. The neuronal and hormonal activities information technology generates regulate many different body functions in a 24-60 minutes wheel. The mouse SCN contains approximately xx,000 neurons.[1]
The SCN interacts with many other regions of the brain. It contains several cell types and several different peptides (including vasopressin and vasoactive intestinal peptide) and neurotransmitters.[ citation needed ]
Neuroanatomy [edit]
The SCN is situated in the anterior role of the hypothalamus immediately dorsal, or superior (hence supra) to the optic chiasm (CHO) bilateral to (on either side of) the tertiary ventricle.
The nucleus can be divided into ventrolateral and dorsolateral portions, likewise known as the core and shell, respectively. These regions differ in their expression of the clock genes, the core expresses them in response to stimuli whereas the shell expresses them constitutively.
In terms of projections, the core receives innervation via iii master pathways, the retinohypothalamic tract, geniculohypothalamic tract, and projections from some Raphe nuclei. Dorsomedial SCN is mainly innervated past the cadre and too by other hypothalamic areas. Lastly, its output is mainly to the subparaventricular zone and dorsomedial hypothalamic nucleus which both mediate the influence SCN exerts over circadian regulation of the body.
Circadian furnishings [edit]
Dissimilar organisms such as bacteria,[2] plants, fungi, and animals, evidence genetically based nearly-24-hour rhythms. Although all of these clocks appear to be based on a similar blazon of genetic feedback loop, the specific genes involved are thought to have evolved independently in each kingdom. Many aspects of mammalian behavior and physiology show circadian rhythmicity, including sleep, physical action, alertness, hormone levels, trunk temperature, allowed office, and digestive activity. The SCN coordinates these rhythms beyond the entire body, and rhythmicity is lost if the SCN is destroyed. For case, full time of sleep is maintained in rats with SCN damage, only the length and timing of slumber episodes becomes erratic. The SCN maintains command beyond the body by synchronizing "slave oscillators," which exhibit their ain about-24-hour rhythms and control circadian phenomena in local tissue.[3]
The SCN receives input from specialized photosensitive ganglion cells in the retina via the retinohypothalamic tract. Neurons in the ventrolateral SCN (vlSCN) have the ability for light-induced factor expression. Melanopsin-containing ganglion cells in the retina have a direct connection to the ventrolateral SCN via the retinohypothalamic tract. When the retina receives low-cal, the vlSCN relays this information throughout the SCN allowing entrainment, synchronization, of the person'south or creature's daily rhythms to the 24-hr bicycle in nature. The importance of entraining organisms, including humans, to exogenous cues such as the light/dark cycle, is reflected past several circadian rhythm sleep disorders, where this procedure does not function normally.[4]
Neurons in the dorsomedial SCN (dmSCN) are believed to take an endogenous 24-60 minutes rhythm that can persist under constant darkness (in humans averaging most 24 hours eleven min).[v] A GABAergic mechanism is involved in the coupling of the ventral and dorsal regions of the SCN.[6]
The SCN sends information to other hypothalamic nuclei and the pineal gland to modulate body temperature and product of hormones such equally cortisol and melatonin.[ citation needed ]
Circadian rhythms of endothermic (warm-blooded) and ectothermic (cold-blooded) vertebrates [edit]

A thermographic prototype of an ectothermic snake wrapping effectually the hand of an endothermic human
Information virtually the straight neuronal regulation of metabolic processes and circadian rhythm-controlled behaviors is not well known amid either endothermic or ectothermic vertebrates, although extensive inquiry has been done on the SCN in model animals such as the mammalian mouse and ectothermic reptiles, in detail, lizards. The SCN is known to be involved not merely in photoreception through innervation from the retinohypothalamic tract but besides in thermoregulation of vertebrates capable of homeothermy, as well as regulating locomotion and other behavioral outputs of the circadian clock within ectothermic vertebrates.[seven] The behavioral differences between both classes of vertebrates, when compared to the respective structures and properties of the SCN and diverse other nuclei proximate to the hypothalamus, provide insight into how these behaviors are the consequence of differing circadian regulation. Ultimately, many neuroethological studies must exist done to completely ascertain the direct and indirect roles of the SCN on circadian-regulated behaviors of vertebrates.
The SCN of endotherms and ectotherms [edit]
In general, external temperature does not influence endothermic animal behavior or circadian rhythm because of the ability of these animals to keep their internal body temperature abiding through homeostatic thermoregulation; however, peripheral oscillators (run across Cyclic rhythm) in mammals are sensitive to temperature pulses and will feel resetting of the circadian clock phase and associated genetic expression, suggesting how peripheral circadian oscillators may be separate entities from one another despite having a master oscillator within the SCN. Furthermore, when private neurons of the SCN from a mouse were treated with heat pulses, a similar resetting of oscillators was observed, but when an intact SCN was treated with the same rut pulse handling the SCN was resistant to temperature change by exhibiting an unaltered circadian aquiver phase.[7] In ectothermic animals, peculiarly the ruin lizard Podacris sicula, temperature has been shown to bear upon the circadian oscillators within the SCN.[eight] This reflects a potential evolutionary relationship amid endothermic and ectothermic vertebrates, in how ectotherms rely on environmental temperature to bear on their circadian rhythms and behavior and endotherms have an evolved SCN to essentially ignore external temperature and employ photoreception as a ways for entraining the circadian oscillators within their SCN. In improver, the differences of the SCN between endothermic and ectothermic vertebrates suggest that the neuronal organization of the temperature-resistant SCN in endotherms is responsible for driving thermoregulatory behaviors in those animals differently from those of ectotherms, since they rely on external temperature for engaging in certain behaviors.
Behaviors controlled by the SCN of vertebrates [edit]
Significant research has been conducted on the genes responsible for controlling circadian rhythm, in detail within the SCN. Knowledge of the gene expression of Clock (Clk) and Period2 (Per2), ii of the many genes responsible for regulating circadian rhythm within the individual cells of the SCN, has allowed for a greater understanding of how genetic expression influences the regulation of circadian rhythm-controlled behaviors. Studies on thermoregulation of ruin lizards and mice have informed some connections between the neural and genetic components of both vertebrates when experiencing induced hypothermic atmospheric condition. Certain findings accept reflected how evolution of SCN both structurally and genetically has resulted in the engagement of feature and stereotyped thermoregulatory behavior in both classes of vertebrates.
- Mice: Among vertebrates, it is known that mammals are endotherms that are capable of homeostatic thermoregulation. Mice have been shown to take some thermosensitivity within the SCN, although the regulation of torso temperature by mice experiencing hypothermia is more sensitive to whether they are in a bright or night surroundings; information technology has been shown that mice in darkened weather and experiencing hypothermia maintain a stable internal torso temperature, even while fasting. In light conditions, mice showed a drop in body temperature nether the same fasting and hypothermic conditions. Through analyzing genetic expression of Clock genes in wild-type and knockout strains, as well as analyzing the activeness of neurons inside the SCN and connections to proximate nuclei of the hypothalamus in the same conditions, it has been shown that the SCN is the middle of control for circadian torso temperature rhythm.[9] This cyclic command, thus, includes both direct and indirect influence of many of the thermoregulatory behaviors that mammals engage in to maintain homeostasis.
- Ruin lizards: Several studies have been conducted on the genes expressed in circadian oscillating cells of the SCN during various light and nighttime atmospheric condition, as well every bit effects from inducing mild hypothermia in reptiles. In terms of structure, the SCNs of lizards have a closer resemblance to those of mice, possessing a dorsomedial portion and a ventrolateral cadre.[ten] However, genetic expression of the circadian-related Per2 factor in lizards is similar to that in reptiles and birds, despite the fact that birds have been known to have a distinct SCN structure consisting of a lateral and medial portion.[eleven] Studying the lizard SCN considering of the cadger'due south pocket-sized body size and ectothermy is invaluable to understanding how this course of vertebrates modifies its beliefs within the dynamics of cyclic rhythm, but information technology has non withal been adamant whether the systems of common cold-blooded vertebrates were slowed equally a result of decreased activity in the SCN or showed decreases in metabolic activity every bit a result of hypothermia.[8]
Other signals from the retina [edit]

A variation of an eskinogram showing the influence of calorie-free and darkness on cyclic rhythms and related physiology and behavior through the SCN in humans
The SCN is one of many nuclei that receive nervus signals directly from the retina.
Some of the others are the lateral geniculate nucleus (LGN), the superior colliculus, the basal optic system, and the pretectum:
- The LGN passes information virtually color, contrast, shape, and movement on to the visual cortex and itself signals to the SCN.
- The superior colliculus controls the motility and orientation of the eye.
- The basal optic system also controls eye movements.[12]
- The pretectum controls the size of the pupil.
Cistron expression [edit]
The cyclic rhythm in the SCN is generated by a factor expression cycle in private SCN neurons. This bike has been well conserved through evolution and in essence is similar in cells from many widely different organisms that evidence circadian rhythms. For example, although fruit flies (like all invertebrates) do not have an SCN, the bike is largely similar to that of mammals. It is currently thought that all animals share a common root in their cyclic rhythm.[13]
Fruitfly [edit]
In the fruitfly Drosophila, the cellular circadian rhythm in neurons is controlled by ii interlocked feedback loops.
- In the start loop, the bHLH transcription factors clock (CLK) and cycle (CYC) drive the transcription of their own repressors period (PER) and timeless (TIM). PER and TIM proteins and then accrue in the cytoplasm, translocate into the nucleus at dark, and plough off their own transcription, thereby setting up a 24-hour oscillation of transcription and translation.
- In the second loop, the transcription factors vrille (VRI) and Pdp1 are initiated past CLK/CYC. PDP1 acts positively on CLK transcription and negatively on VRI.
These genes encode various transcription factors that trigger expression of other proteins. The products of clock and bicycle, called CLK and CYC, belong to the PAS-containing subfamily of the basic helix-loop-helix (bHLH) family of transcription factors, and form a heterodimer. This heterodimer (CLK-CYC) initiates the transcription of PER and TIM, whose protein products dimerize and then inhibit their own expression past disrupting CLK-CYC-mediated transcription. This negative feedback mechanism gives a 24-hour rhythm in the expression of the clock genes. Many genes are suspected to be linked to circadian command by "E-box elements" in their promoters, as CLK-CYC and its homologs bind to these elements.
The 24-hour rhythm could exist reset past lite via the protein cryptochrome (Weep), which is involved in the cyclic photoreception in Drosophila. CRY assembly with TIM in a light-dependent fashion that leads to the destruction of TIM. Without the presence of TIM for stabilization, PER is somewhen destroyed during the day. Every bit a result, the repression of CLK-CYC is reduced and the whole cycle reinitiates once again.
Mammals [edit]
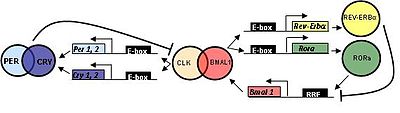
The oscillator genes and proteins involved in the mammalian circadian oscillator
In mammals, cyclic clock genes behave in a way similar to that of flies.
CLOCK (circadian locomotor output cycles kaput) was first cloned in mouse and BMAL1 (brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like 1) is the primary homolog of Drosophila CYC.
Three homologs of PER (PER1, PER2, and PER3) and two Weep homologs (CRY1 and CRY2) take been identified.
TIM has been identified in mammals; nevertheless, its office is withal non determined. Mutations in TIM result in an disability to reply to zeitgebers, which is essential for resetting the biological clock.[ citation needed ]
Recent research suggests that, outside the SCN, clock genes may have other important roles likewise, including their influence on the furnishings of drugs of corruption such equally cocaine.[fourteen] [15]
Electrophysiology [edit]
Neurons in the SCN fire action potentials in a 24-hour rhythm. At mid-24-hour interval, the firing rate reaches a maximum, and, during the night, it falls again. How the gene expression cycle (so-called the cadre clock) connects to the neural firing remains unknown.[ commendation needed ]
Many SCN neurons are sensitive to calorie-free stimulation via the retina, and sustainedly firing action potentials during a light pulse (~30 seconds) in rodents. The photic response is likely linked to effects of light on circadian rhythms. In addition, focal application of melatonin can decrease firing activeness of these neurons, suggesting that melatonin receptors nowadays in the SCN mediate stage-shifting furnishings through the SCN.[ commendation needed ]
See also [edit]
- Chronobiology
- Photosensitive ganglion prison cell
- Sense of fourth dimension
- Retinohypothalamic tract
- Shift work sleep disorder
- Non-24-hour sleep–wake disorder
References [edit]
- ^ Fahey J (2009-10-fifteen). "How Your Brain Tells Time". Out Of The Labs. Forbes.
- ^ Clodong S, Dühring U, Kronk Fifty, Wilde A, Axmann I, Herzel H, Kollmann Yard (2007). "Operation and robustness of a bacterial circadian clock". Molecular Systems Biological science. 3 (1): 90. doi:x.1038/msb4100128. PMC1847943. PMID 17353932.
- ^ Bernard S, Gonze D, Cajavec B, Herzel H, Kramer A (April 2007). "Synchronization-induced rhythmicity of circadian oscillators in the suprachiasmatic nucleus". PLOS Computational Biology. 3 (iv): e68. Bibcode:2007PLSCB...3...68B. doi:10.1371/journal.pcbi.0030068. PMC1851983. PMID 17432930.
- ^ Reid KJ, Chang AM, Zee PC (May 2004). "Cyclic rhythm slumber disorders". The Medical Clinics of North America. 88 (3): 631–51, viii. doi:10.1016/j.mcna.2004.01.010. PMC3523094. PMID 15087208.
- ^ "Homo Biological Clock Prepare Back an Hour". Harvard Gazette. 1999-07-15. Retrieved 2019-01-28 .
- ^ Azzi, A; Evans, JA; Leise, T; Myung, J; Takumi, T; Davidson, AJ; Dark-brown, SA (18 Jan 2017). "Network Dynamics Mediate Circadian Clock Plasticity". Neuron. 93 (2): 441–450. doi:10.1016/j.neuron.2016.12.022. PMC5247339. PMID 28065650.
- ^ a b Buhr ED, Yoo SH, Takahashi JS (October 2010). "Temperature equally a universal resetting cue for mammalian circadian oscillators". Science. 330 (6002): 379–85. Bibcode:2010Sci...330..379B. doi:10.1126/science.1195262. PMC3625727. PMID 20947768.
- ^ a b Magnone MC, Jacobmeier B, Bertolucci C, Foà A, Albrecht U (February 2005). "Circadian expression of the clock factor Per2 is altered in the ruin cadger (Podarcis sicula) when temperature changes" (PDF). Encephalon Inquiry. Molecular Brain Research. 133 (2): 281–five. doi:ten.1016/j.molbrainres.2004.10.014. PMID 15710245.
- ^ Tokizawa Thousand, Uchida Y, Nagashima Thou (December 2009). "Thermoregulation in the common cold changes depending on the fourth dimension of day and feeding condition: physiological and anatomical analyses of involved circadian mechanisms". Neuroscience. 164 (3): 1377–86. doi:10.1016/j.neuroscience.2009.08.040. PMID 19703527. S2CID 207246725.
- ^ Casini G, Petrini P, Foà A, Bagnoli P (1993). "Blueprint of system of primary visual pathways in the European lizard Podarcis sicula Rafinesque". Periodical für Hirnforschung. 34 (3): 361–74. PMID 7505790.
- ^ Abraham U, Albrecht U, Gwinner E, Brandstätter R (August 2002). "Spatial and temporal variation of passer Per2 gene expression in ii distinct cell groups of the suprachiasmatic hypothalamus in the firm sparrow (Passer domesticus)". The European Journal of Neuroscience. 16 (3): 429–36. doi:10.1046/j.1460-9568.2002.02102.ten. PMID 12193185. S2CID 15282323.
- ^ Giolli RA, Blanks RH, Lui F (2006). "The accompaniment optic system: basic organization with an update on connectivity, neurochemistry, and office" (PDF). Progress in Brain Inquiry. 151: 407–40. doi:10.1016/S0079-6123(05)51013-6. ISBN9780444516961. PMID 16221596.
- ^ Young, Michael Westward.; Kay, Steve A. (September 2001). "Time zones: a comparative genetics of circadian clocks". Nature Reviews Genetics. two (9): 702–715. doi:10.1038/35088576.
- ^ Yuferov V, Butelman ER, Kreek MJ (October 2005). "Biological clock: biological clocks may attune drug habit". European Journal of Human Genetics. xiii (10): 1101–three. doi:10.1038/sj.ejhg.5201483. PMID 16094306. S2CID 26531678.
- ^ Manev H, Uz T (January 2006). "Clock genes as a link between addiction and obesity". European Journal of Man Genetics. 14 (one): 5. doi:ten.1038/sj.ejhg.5201524. PMID 16288309.
External links [edit]
- Diagram at thebrain.mcgill.ca
williamssquess1940.blogspot.com
Source: https://en.wikipedia.org/wiki/Suprachiasmatic_nucleus
0 Response to "what happens after damage to the suprachiasmatic nucleus itself?​"
Post a Comment